Greetings from all of us here at UHMS!
As 2024 draws to a close, I am humbled by the tremendous progress made in advancing the undersea and hyperbaric medicine specialty. Thanks to our members' and volunteers' unwavering support and dedication, we’ve strengthened our community, expanded our reach (a bigger tent), and furthered our mission to promote the safe and effective practice of hyperbaric medicine.
Here are some key highlights of our collective accomplishments this year:
1. Strengthening Education and Certification Pathways
UHMS's directly sponsored and jointly provided educational programming continues to be the cornerstone of our specialty. It serves the spectrum of learning from basic to advanced using all learning modes, from in-person meetings to enduring material programs.
Learners worldwide attended our programming this year, and the UHMS experienced record attendance at many of our meetings and courses. This demonstrates that members of our specialty are thirsty for knowledge and committed to lifelong learning.
We are working to strengthen our certification programs by formalizing the continuous certification process around each certification pathway. This should be complete by the end of Q1 2025.
One of the certification programs is the UHMS Physician Training in Diving Medicine Course (PTDM), held in La Jolla, CA. The UHMS has hosted this DMO course for over 40 years, and according to Dr. Simon Mitchell, it is the best diving medicine course in the world.
I was honored to be invited to attend and participate in the diving practicum day. While I have attended different parts of the course over the years, this was the first time I witnessed the ‘magic’ that happens during this full day of in-water diving practicums. Stations included extracting unconscious divers from the water, navigating an underwater obstacle course on SCUBA with body (manikin) retrieval, rebreather orientation and diving, diving skills and checklists, and more. Hats off to Drs. Pete Witucki and Dick Sadler for planning and executing another stellar program, as well as for all the faculty. In addition, we’d like to express our heartfelt appreciation for Spencer Lynch and the LFJCC for the use of their aquatic center, Oceanside Lifeguards Blake Faumuina and Greg Trebbe, technical dive instructor Jay Gardner, rebreather instructor Todd Wynn, and Drs. Dan Popa and Christanne Coffey.

 I appreciate the quote from Abigail Adams, the wife of POTUS No. 2, John Adams; “Learning is not attained by chance, it must be sought for with ardor and diligence.” Data shows that education, experience, and certification are essential differentiators from a practice and patient outcomes perspective.
I appreciate the quote from Abigail Adams, the wife of POTUS No. 2, John Adams; “Learning is not attained by chance, it must be sought for with ardor and diligence.” Data shows that education, experience, and certification are essential differentiators from a practice and patient outcomes perspective.
2. Expanding Public and Professional Awareness
Raising awareness of the vital role hyperbaric medicine plays in healthcare has been a key focus this year.
3. Advocating for Broader Access to Care
Our advocacy efforts have yielded meaningful progress in ensuring HBO2 is accessible and recognized as an essential treatment modality.
- Insurance Coverage Wins: We partnered with stakeholders to secure expanded coverage for HBO2 to treat the late effects of radiation therapy, compromised skin grafts and flaps, avascular necrosis (AVN), and other conditions.
- Policy Advocacy: We successfully engaged with the Alliance of Wound Care Stakeholders and federal and state policymakers to emphasize hyperbaric medicine's cost-effectiveness and efficacy compared to standard care.
4. Advancing Research and Innovation
UHMS has continued to lead in driving evidence-based advancements in hyperbaric medicine.
- Research:UHMS continues to fund research and data analysis to validate existing diagnoses as well as emerging indications, like inflammatory bowel disease and Crohn's, traumatic brain injury, and post-COVID recovery.
- Publications: We published numerous papers on the cost-effectiveness and efficacy of hyperbaric medicine, solidifying our role as a trusted authority in the field.
5. Enhancing Member Services and Global Reach
Membership is the core of the UHMS, and we continue to be hyperfocused on creating value for members and Corporate Partners.
Looking Ahead to 2025
While we celebrate these accomplishments, we know much work still needs to be done. In 2025, our focus continues to be mission, mission, mission.
~
I will return to my soapbox momentarily to repeat my message from the Q1 issue of Pressure.
We need your help. The physician, advanced provider, nurse, therapist, and technician should take responsibility for seeking and maintaining certification and completing 12 hours of continuing education annually. Hospitals, management providers, and chamber manufacturers are accountable for supporting and promoting hyperbaric facility accreditation and providing urgent and emergent hyperbaric medicine care for all patients.
This is a call to action for everyone involved in hyperbaric medicine: to get engaged, get educated, get certified, get your facility accredited, and show that you're doing what you claim to do, particularly around delivering optimal outcomes and treating patients of all acuities regardless of payor source.
Please review our position statement on Certification Matters: UHMS POSITION STATEMENT.
The UHMS tent is wide open, so please come in and join us to make our specialty vibrant and viable for generations to come!
UHMS Finances
I am pleased to report that UHMS's Financial position remains strong.
| Jan-Oct 2024 PL |
| |
Actual |
Budget |
| Income |
$1,505,236 |
$1,438,851 |
| Expense |
$1,251,053 |
$1,380,893 |
| Net |
$254,182 |
$57,958 |
Our balance sheet remains healthy, with operating, savings, and investment accounts continuing to hover at near-all-time highs.
Member Benefits
As a reminder, UHMS members receive three free CE/CME credits upon joining or renewing. This benefit represents an immediate $40 savings for Associate members and $60 for Regular members annually.
Associate Member Town Hall
Members are invited to attend the UHMS Associate Council town hall meeting on the second Thursday of every quarter, where invited speakers present on relevant topics that apply to our specialty.
Corporate Partners
If you are a UHMS Corporate Partner, please attend our monthly Corporate Partner Town Hall meeting series. These are held on the 1st Wednesday of every month at 12 PM and are intended to be an open forum for discussing the challenges and successes your businesses and practices are experiencing and to create momentum and collaboration where appropriate.
If your organization wants to educate the UHMS membership about the care provided or the goods and services offered, consider joining our Corporate Partnership Program. For more information, visit https://www.uhms.org/corporate-memberships.html.
MEDFAQs
The UHMS offers its version of "ask the experts." MEDFAQs can be found at the following URL – https://www.uhms.org/resources/medfaqs-frequently-asked-questions-faq.html, and is a valuable tool for our membership.
If you are familiar with MEDFAQs, check back, as new Q&As are posted regularly.
Research
The UHMS Research Committee continues to be very active. We hope to announce some good news in the new year on the IRB front, where members of our community can come to the UHMS for the Institutional Review Board's needs.
We are soliciting donations from our members for two research initiatives. One is the Continuous Glucose Monitor study (https://www.uhms.org/cgm-hyperbaric-oxygen-study), and the Multicenter Registry for Hyperbaric Oxygen Therapy at Dartmouth (MRHBO2) continues seeking funds for free hospital membership. The MRHBO2 is funded entirely via grants, not by the registry's participating hospitals - https://www.uhms.org/donate-to-the-multicenter-registry-for-hyperbaric-oxygen-therapy.html.
Remember that donations to the UHMS Funds for Research and Policy Advancement are tax-deductible. For more information, visit the UHMS website at https://www.uhms.org/funding.html.
QUARC
To better understand the field's challenges, log in and visit the QUARC page at https://www.uhms.org/resources/quarc.html. Here, you will find impending legislation and other relevant policies on the provision and limitations of HBO2 coverage, as well as the UHMS's responses and guidance.
The chairs of QUARC request that you let us know as soon as possible about any unusual denials or challenges with physicians gaining access to insurance panels for HBO2 services. jpeters@uhms.org.
UHM
If you are a UHMS member, we are happy to announce a new search feature for previous issues and articles from UHM/UBR - https://www.uhms.org/publications/uhm-journal/download-uhm-journal-pdfs.html. Currently, the feature works with keywords.
If you have a suggestion or comment on how we can better serve you, please email me at jpeters@uhms.org or call at 561-776-6110 extension 100.
As always, our success is a testament to the dedication and passion of our members. Thank you for your continued contributions to advancing our field and improving patient outcomes worldwide.
Together, we are making a difference, one breath at a time.
John Peters




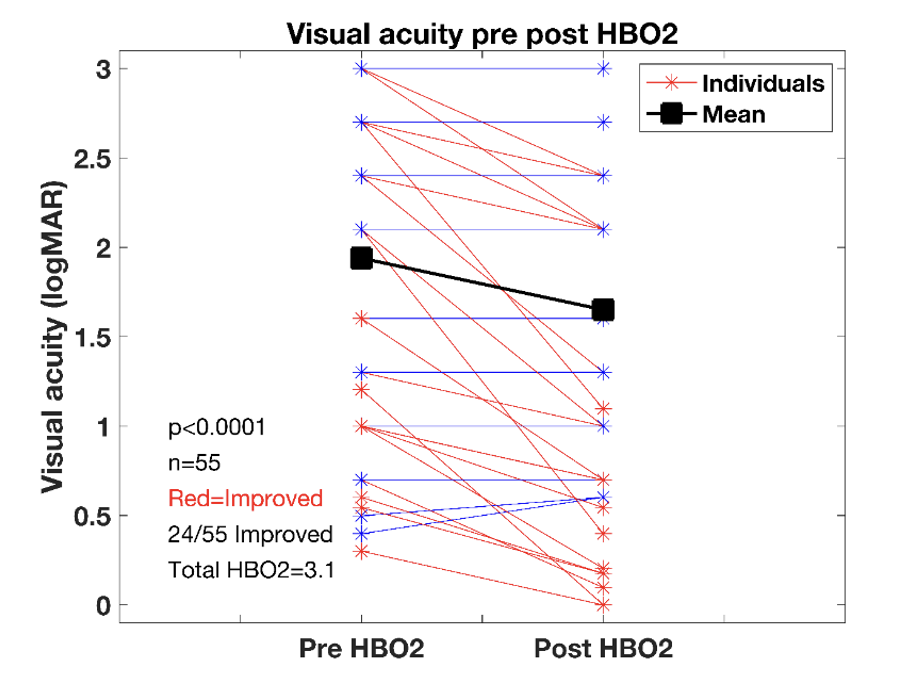 The registry data show that patients treated at Registry centers have, on average, an improvement in vision. The Registry currently includes 55 eyes with CRAO or branch retinal artery occlusion (BRAO), who received HBO2 the same day as their initial visual acuity measurement and had at least one treatment. This is twice as many people in the Kalaw et al. study. Figure presents pre- and post-visual acuity measurements in this cohort and shows that 44% of patients regained visual acuity, similar to what is seen in other studies.
The registry data show that patients treated at Registry centers have, on average, an improvement in vision. The Registry currently includes 55 eyes with CRAO or branch retinal artery occlusion (BRAO), who received HBO2 the same day as their initial visual acuity measurement and had at least one treatment. This is twice as many people in the Kalaw et al. study. Figure presents pre- and post-visual acuity measurements in this cohort and shows that 44% of patients regained visual acuity, similar to what is seen in other studies.